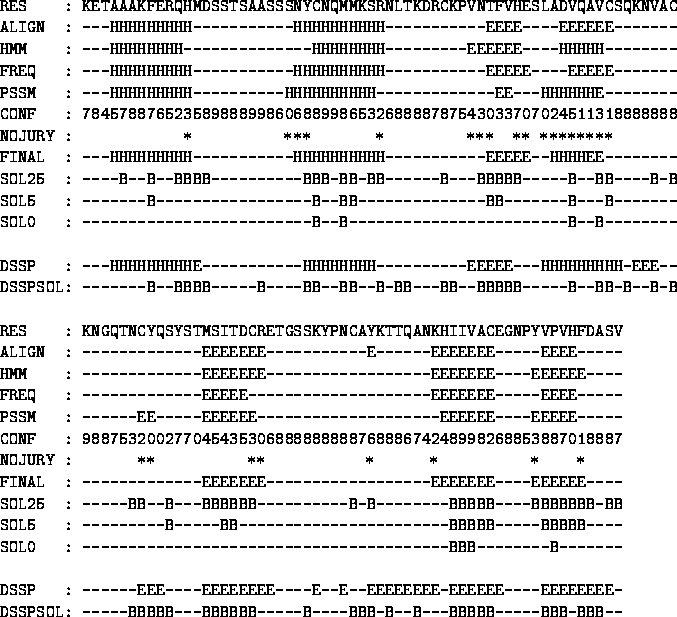
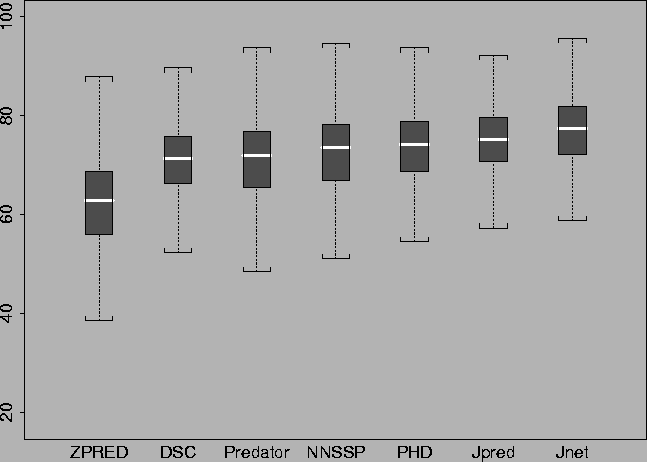
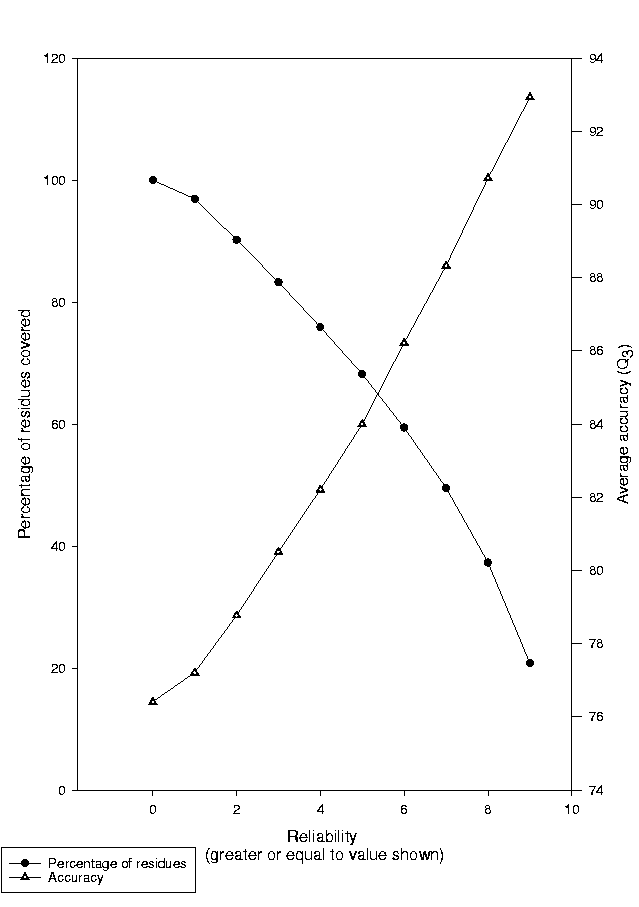
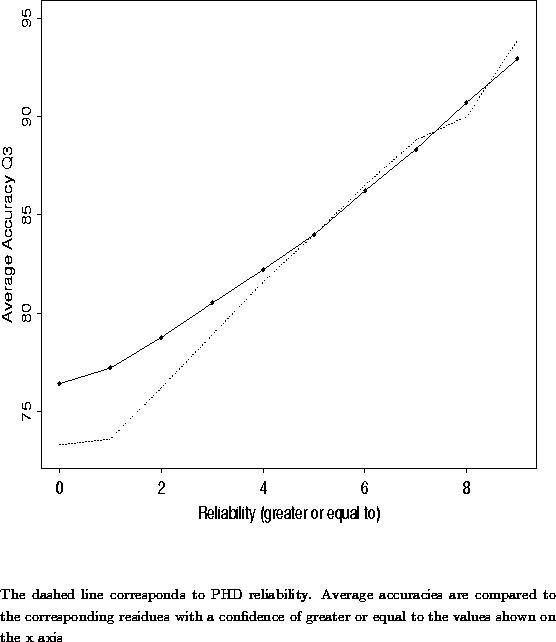
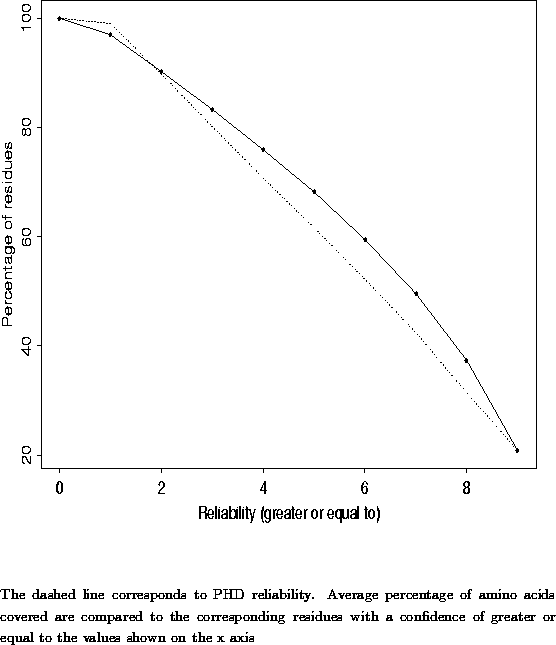
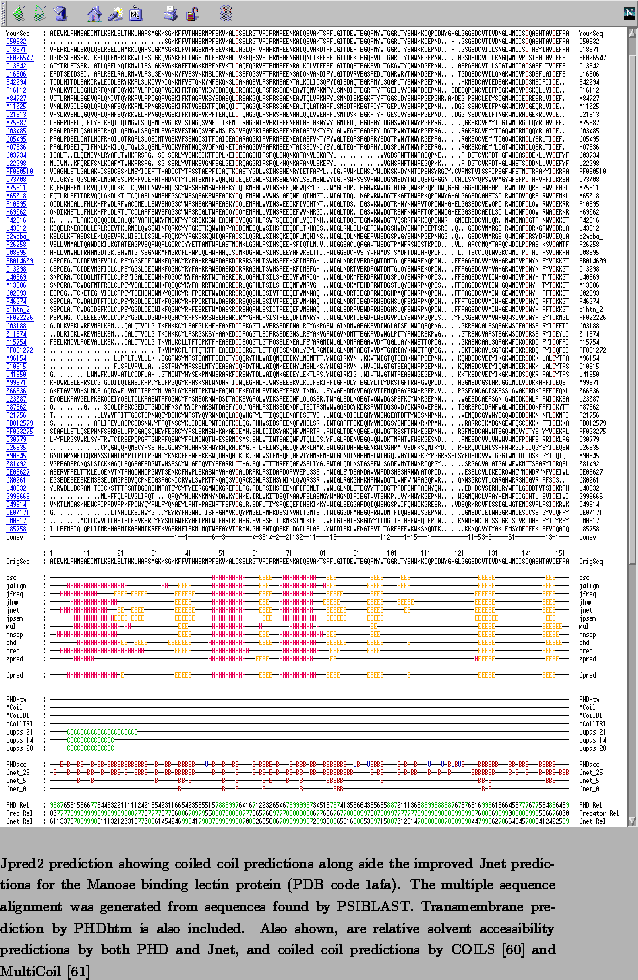
A new approach to protein fold recognition.
Nature, 358:86-89, 1992.
Protein fold recognition by prediction-based threading.
J. Mol. Biol., 270:1-10, 1997.
Protein fold recognition by mapping predicted secondary structures.
J. Mol. Biol., 259:349-365, 1996.
Recognition of analogous and homologous protein folds: Analysis of sequence and structure conservation.
J. Mol. Biol., 269:423-439, 1997.
Protein fold recognition using sequence-derived potentials.
Prot. Sci., 5:947-955, 1996.
Prediction of protein secondary structure and active sites using the alignment of homologous sequences.
J. Mol. Biol., 195:957-961, 1987.
Analysis and implications of simple methods for predicting the secondary structure of globular proteins.
J. Mol. Biol., 120:97-120, 1978.
Conformational parameters for amino acids in helical,
Biochem., 13:211-222, 1974.
GOR method for predicting protein secondary structure from amino acid sequence.
Meth. Enz., 266:540-553, 1996.
Further developments of protein secondary structure prediction using information theory. New parameters and consideration of residue pairs.
J. Mol. Biol., 198:425-443, 1987.
Predicting protein secondary structure based on amino acid sequence.
Meth. Enz., 202:31-44, 1995.
Algorithms for prediction of
J. Mol. Biol., 88:873-894, 1974.
Identification and application of the concepts important for accurate and reliable protein secondary structure prediction.
Prot. Sci., 5:2298-2310, 1996.
Machine learning approach for the prediction of protein secondary structure.
J. Mol. Biol., 216:441-457, 1990.
Protein secondary structure prediction using logic-based machine learning.
Prot. Eng., 5:647-657, 1992.
Predicting the secondary structure of globular proteins using neural network models.
J. Mol. Biol., 202:865-884, 1988.
Prediction of protein secondary structure at better than 70% accuracy.
J. Mol. Biol., 232:584-599, 1993.
Protein secondary structure prediction with a neural network.
Proc. Nat. Acad. Sci., 86:152-156, 1989.
Improvements in protein secondary structure prediction by an enhanced neural network.
J. Mol. Biol., 1:171-182, 1990.
Improving prediction of protein secondary structure using structured neural networks and multiple sequence alignments.
J. Comput. Biol., 1:163-183, 1996.
The importance of larger data sets for protein secondary structure prediction with neural networks.
Protein Sci., 5:768-774, 1996.
New methods for accurate prediction of protein secondary structure.
Proteins, 35:293-306, 1999.
Prediction of protein secondary structure by combining nearest- neighbor algorithms and multiple sequence alignments.
J. Mol. Biol., 247:11-15, 1995.
Secondary structure prediction using segment similarity.
Prot. Eng., 10:1143-1153, 1997.
Incorporation of non-local interactions in protein secondary structure prediction from the amino acid sequence.
Prot. Eng., 9:133-142, 1996.
Protein secondary structure prediction using nearest-neighbor methods.
J. Mol. Biol., 232:1117-1129, 1993.
Exploring the limits of nearest neighbour secondary structure prediction.
Prot. Eng., 10:771-776, 1997.
Sopma : Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments.
Comp. App. Biosci., 11:681-684, 1995.
Using evolutionary trees in protein secondary structure prediction and other comparative sequence analyses.
J. Mol. Biol., 263:196-208, 1996.
PASSML: combining evolutionary inference and protein secondary structure prediction.
Bioinformatics, 8:726-733, 1998.
A simple and fast approach to prediction of protein secondary structure from multiply aligned sequences with accuracy above 70%.
Prot. Sci., 4:2517-2525, 1995.
In unison: regularization of protein secondary structure predictions that makes use of multiple sequence alignments.
Prot. Eng., 10:861-865, 1998.
New joint prediction algorithm (Q7-JASEP) improves the prediction of protein secondary structure.
Biochemistry, 46:11164-11172, 1991.
Evaluation and improvement of multiple sequence methods for protein secondary structure prediction.
Proteins: Structure, Function and Genetics, 34:508-519, 1999.
Secondary structure prediction: combination of three different methods.
Prot. Eng., 2:185-191, 1995.
Improved performance in protein secondary structure prediction by inhomogeneous score combination.
Bioinformatics, 5:413-421, 1999.
Critical assesment of methods of protein structure prediction (CASP): Round II.
Proteins, Suppl. 1:1-230, 1997.
Third Meeting on the Critical Assesment of Techniques for Protein Structure Prediction: Asilomar Conference Centre, Dec ember 13-17.
http://predictioncenter.llnl.gov/casp3/Casp3.html, 1998.
Prediction of protein secondary structure at 77% accuracy based on PSIBLAST derived sequence profiles.
Third Meeting on the Critical Assesment of Techniques for Protein Structure Prediction: Asilomar Conference Centre, December 13-17, 1998.
Gapped BLAST and PSI-BLAST: a new generation of protein database search programs.
Nuc. Ac. Res., 25:3389-3402, 1997.
Sequence comparisons using multiple sequences detect three times as many remote homologues as pairwise methods.
J. Mol. Biol., 284:1201-1210, 1998.
Protein multiple sequence alignment and flexible pattern matching.
Meth. Enz., 183:403-428, 1990.
The SWISS-PROT protein sequence data bank and its supplement TrEMBL in 1999.
Nucleic Acids Res., 27:49-54, 1998.
CLUSTAL W: improving the sesitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice.
Nuc. Ac. Res., 22:4673-4680, 1994.
HMMer2.
http://hmmer.wustl.edu/, 1999.
Hidden markov models in computational biology.
J. Mol. Biol., 235:1501-1531, 1994.
Biological sequence analysis.
Cambridge University Press, 1998.
Statistics of local complexity in amino acid sequences and sequence databases.
Comput. Chem, 17:149-163, 1993.
A model recognition approach to the prediction of all-helical membrane protein structure and topology.
Biochemistry, 33:3038-3049, 1994.
The SNNS users manual version 4.1.
http://www.informatik.uni-stuttgart.de/ipvr/bv/projekte/snns/UserManual/User Manual.html, 1995.
A scaled conjugate gradient algorithm for fast supervised learning.
Neural Networks, 6:525-533, 1993.
A dictionary of protein secondary structure.
Biopolymers, 22:2577-2637, 1983.
The hydrophobicity profile.
In G. D. Fasman, editor, Prediction of protein structure and the principles of protein conformation, chapter 15, pages 625-634. Plenum press, 233 Spring street, New York, NY, 10013, 1989.
Redefining the goals of protein secondary structure prediction.
J. Mol. Biol., 235:13-26, 1994.
Jpred: a consensus secondary structure prediction server.
Bioinformatics, 14:892-893, 1998.
A modified definition of SOV, a segment-based measure for protein secondary structure prediction assesment.
Proteins, 34:220-223, 1999.
The protein data bank: A computer based archival file for macromolecur structures.
J. Mol. Biol., 112:535-542, 1977.
The refined crystal structure of ribonuclease A at 2.0 A resolution.
J. Biol. Chem., 257:1325-1332, 1982.
Variations of box plots.
The American Statistician, 32:12-16, 1978.
Predicting coiled coils from protein sequences.
Science, 252:1162-1164, 1991.
MultiCoil: a program for predicting two- and three-stranded coi led coils.
Prot. Sci., 6:1178-1189, 1997.
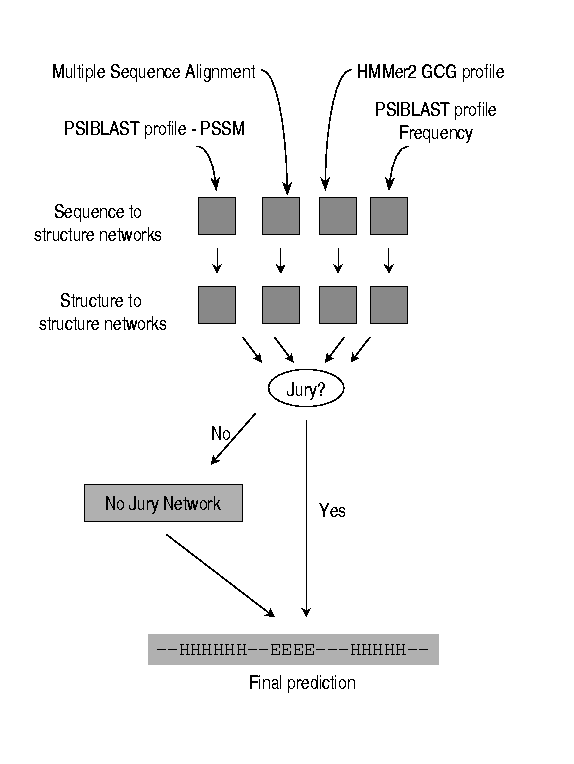
![\begin{landscape}% latex2html id marker 226
\begin{figure}[h]
\begin{center}
\le...
...ed proteins from a profile based search.}\end{center}\end{figure}\end{landscape}](img7.png)